ASTM F1980
Standard Guide for Accelerated Aging of Sterile Barrier Systems and Medical Devices
 ASTM F1980 is also known as the Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices. It offers multiple aging protocols to determine the effects of time on the sterile integrity of the packaging as well as the physical properties of the latter. While this guide does evaluate the sterile barrier system shelf life, it does not address the barrier system material and device interaction compatibility. Micom offers ASTM F1980 as part of its Medical devices testing services.
ASTM F1980 is also known as the Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices. It offers multiple aging protocols to determine the effects of time on the sterile integrity of the packaging as well as the physical properties of the latter. While this guide does evaluate the sterile barrier system shelf life, it does not address the barrier system material and device interaction compatibility. Micom offers ASTM F1980 as part of its Medical devices testing services.
It is important to note that the 2016 version of ASTM F1980 used to state in section 6.5: “A humidity factor to calculate the accelerated aging time(AAT) is not applicable for accelerated aging protocols.” The 2021 version of the standard now states, in the same section: “Since sterile barrier systems and medical device are stored in environments that comprise varying levels of ambient humidity, and since the properties of some materials may depend on the level of absorbed moisture […] it is important to consider not only the accelerated aging temperature conditions but also the ambient relative humidity during that accelerated aging.” This change is extremely important as some of the chemical reactions occurring during the heat aging of some materials require the presence of ambient humidity. In other words, for some materials, having done a heat aging test without added moisture may have prevented the occurrence of deleterious chemical reactions that might have further damaged some of the barrier systems and/or medical devices materials.
Micom offers ASTM F1980 as part of its Medical devices testing services.
Uses and Factors to Be Considered
The information derived from testing to this guide is considered adequate in establishing expiration dates for both medical devices and sterile barriers while real-time aging studies are being conducted.
The aging of products or materials refers to the variation of their properties over time. The properties of interest are those related to safety and efficacy. An aging study is a procedure that seeks the determination of the behavior of a device under normal-usage conditions over a relatively long time by exposing the material for a short period of time to external stress, which is more severe or more frequently applied than normal environmental stresses. Many accelerated aging techniques used for the qualification testing of polymer medical devices are based on the assumption that the chemical reactions involved in the deterioration of materials follow the Arrhenius’ law. According to the latter, aging processes increase as a function of the ambient temperature. This function states that any chemical reaction will double its rate with each 10 oC increase. This ideal case would correspond to curve Q10=2 below. However, the value of the Q10 is not always “2”; using the Arrhenius reaction rate function with Q10 equal to two is a common and conservative way of calculating the aging factor. For additional information about Arrhenius law, please see; Heat Aging Testing.
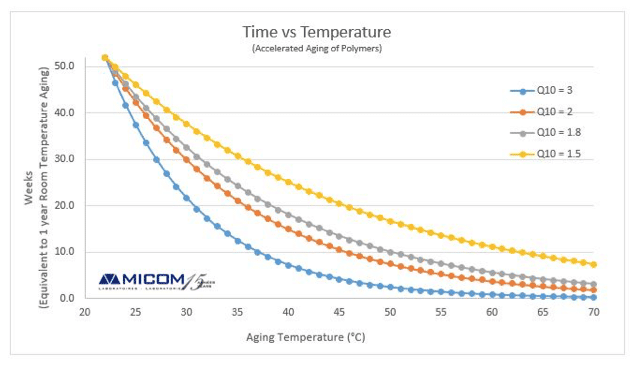
Medical devices need to be able to be stored for an extended period without any decrease in performance that may influence safety and efficacy when used. The ability of product designers to predict in a precise way the changes in polymer properties is therefore crucial and this standard guide provides sufficient evidence for claimed expiry date until data from real-time aging studies are available. The loss of sterile barrier system integrity can be the result of the deterioration of physical properties of the materials, the degradation of adhesive or cohesive bonds as well as the subsequent dynamic events during shipping and handling. Furthermore, a given polymer may have many functional chemical groups organized in various ways (glass, amorphous, crystalline) as well as additives, processing agents, catalysts, lubricants, residual solvents, corrosive gases and fillers. These elements combined with variations in end-use conditions and storage environment influence the degradation chemistry. However, ISO 11607-1:2006 states that “the packaging system must provide physical protection, maintain sterile conditions and integrity of the sterile barrier system to the point of use or until the expiry date.”
Typical Experimental Parameters for ASTM F1980 Testing
- Number of specimens/products: to be specified
- Room or ambient temperature: Must represent the actual product storage and use conditions. Typically between 20oC and 25oC.
- Accelerated aging temperature: To be specified, but typically below 60oC since higher temperature may induce nonlinear changes.
- Accelerated aging factor: To be specified. It is calculated by the following equation:
![]()
Where:
TAA: Accelerated aging temperature (oC)
TRT: Ambient temperature (oC)
Q10: Rate of the chemical reaction, typically 2
- Humidity conditions: Various materials will be impacted differently by the presence of ambient humidity. Section X3.3 of ASTM F1980 states that “When controlling humidity during an accelerated aging study, 45-55 % RH should be targeted unless there is specific warrant for using other humidity conditions (based on material knowledge). If other conditions are used, a rationale should be documented.”
Post-Aging Evaluation
The sterile barrier systems that have been subjected to aging are evaluated for both physical properties and integrity to assess whether or not product properties were modified or adversely compromised compared to the unexposed ones. Mechanical tests such as tensile properties and other physical tests tailored to your specific needs should be considered at different time intervals during the accelerated aging process.
Other Test Methods Related to ASTM F1980
For additional test methods related to ASTM F1980 we invite you to read on Accelerated aging and ASTM D3045
If you have any questions about the ASTM F1980 test or other material tests, we invite you to contact our material testing laboratory. We will be happy to answer your questions and help you with your testing requirements.
Accelerated Aging Calculator
Results:
Accelerated Aging Factor (AAF):
AAT (Testing Time):
Days:
Weeks:

